
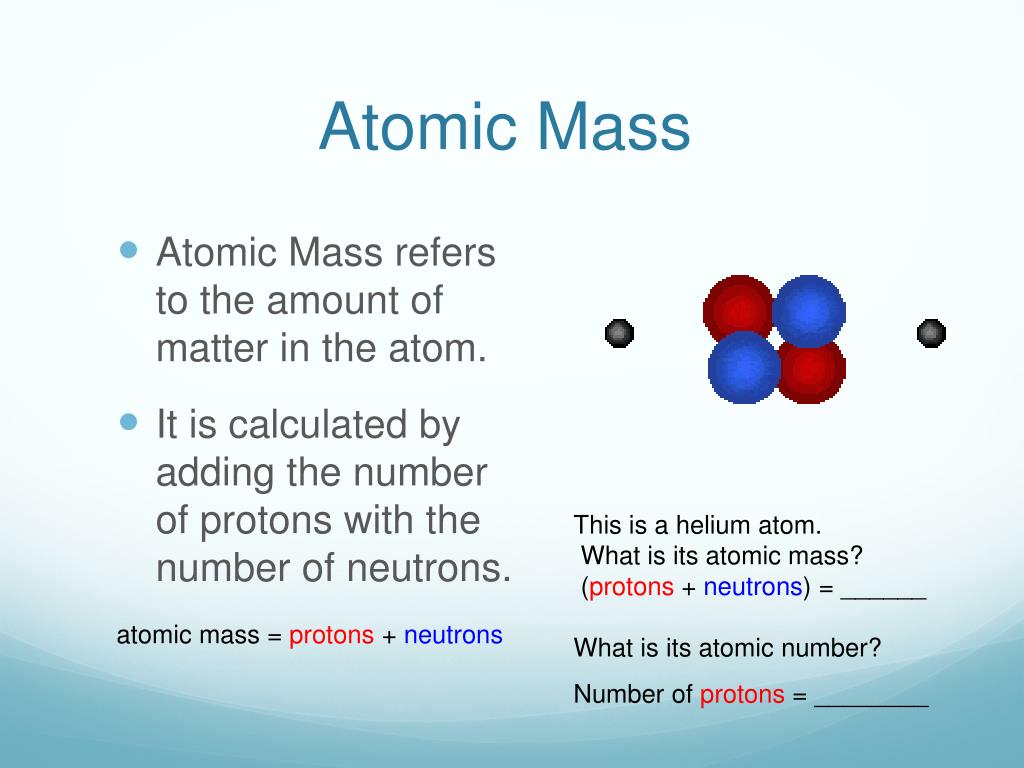
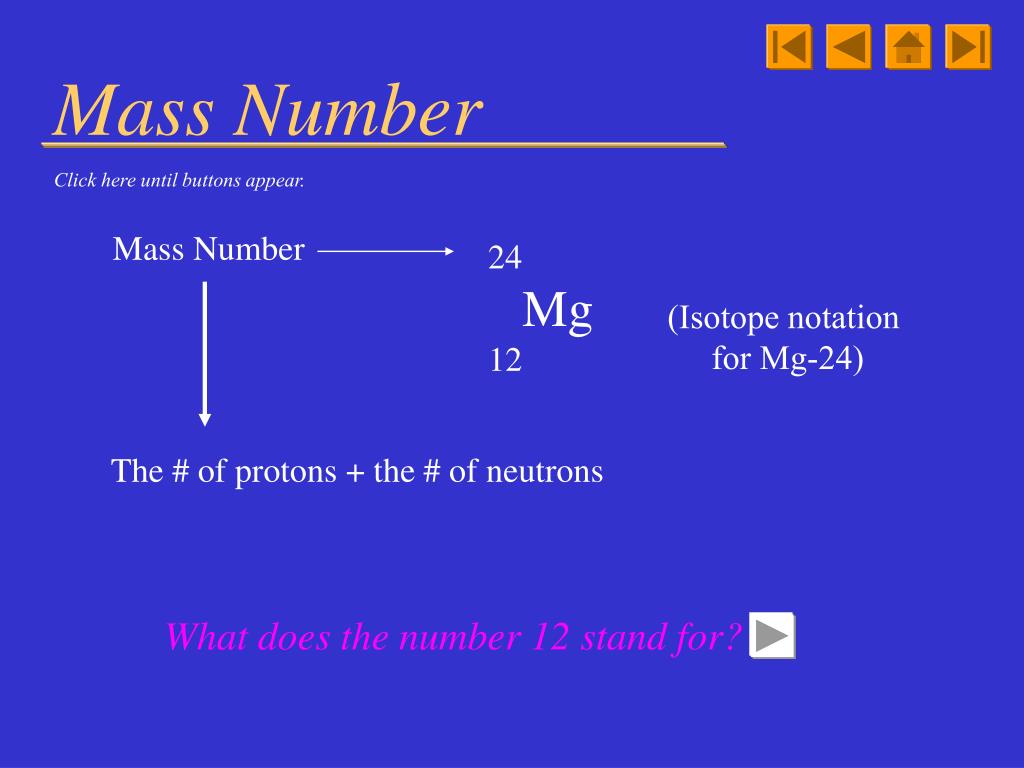
See the legend below the table for more details. What is Atomic mass Calculator The Atomic mass is the mass of a molecule, including its protons, Atoms, and electrons. When you click an element tile of interest within this periodic table, you will see additional details and important properties of the element, and be able to select for your experiments various element forms, compounds and, in the case of metals, alloys. Relative atomic mass definition: the ratio of the average mass per atom of the naturally occurring form of an element to. Easy-to-use filters allow you to sort by metals, nonmetals, physical states, group, period, and more. Find Atomic Mass for a Single Atom Since the combined masses of protons and neutrons account for almost all the mass of the given atom, the atomic mass of a single atom can be calculated by adding the total number of protons and the total number of neutrons of that particular isotope. Elements with the same number of valence or outer shell electrons are placed in vertical columns called groups and labeled from 1 to 18. This ground state configuration is the key to understanding an element’s chemistry. 7 Our carbon atom has 6 protons + 6 neutrons 12. The 21st century version of Mendeleev’s original creation also recognizes that periodicity is a consequence of the variation in the elements’ ground state electron configurations, i.e., the number and arrangement of electrons. To find the atomic mass of a given element or an isotope of an element, there are 3 steps involved: Method 1 Sum total of protons and neutrons of a single atom These steps involve calculating the sum of the total number of protons and the total number of neutrons. Don't worry about the number of electrons orbiting the nucleus - their combined mass is very, very small, so, in most practical cases, it won't significantly affect your answer. As more elements were discovered, they were arranged in the periodic table in numerical order according to their atomic number, or the number of protons (or electrons) they possessed. See for instance here for further details.The periodic table of the elements was first introduced in the mid-19th century by Dmitri Mendeleev, who originally represented the elements as periodic functions of their atomic weights. Takes in grams or (m) of an element and dividing it by its atomic mass (amu) its able to find the limiting reactant. Because the binding of the neutrons and protons is much stronger than the binding energy of the electrons to the core, the effect of the mass is also much larger. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation. In the same way that the formation of a hydrogen atom from an electron and a proton lowers the mass of the atom by the corresponding binding energy, the binding energy between the neutrons, protons and electrons that make up heavier atoms also results in a reduction of the mass of the atom. Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons.
The principles of operation of a modern mass spectrometer are studied. In this scale, 1 atomic mass unit (amu) corresponds to 1.660539040 × 10 24 gram.
It is expressed as a multiple of one-twelfth the mass of the carbon -12 atom, 1.992646547 × 10 23 gram, which is assigned an atomic mass of 12 units. In simplest terms, there is one isotope, $\ce$ J or $-13.7$ eV which is very close to the Rydberg constant! Chemists can measure the mass of atoms and molecules to a high degree of accuracy in a mass spectrometer. atomic mass, the quantity of matter contained in an atom of an element.

This is a simple question which has a complicated answer.


 0 kommentar(er)
0 kommentar(er)
